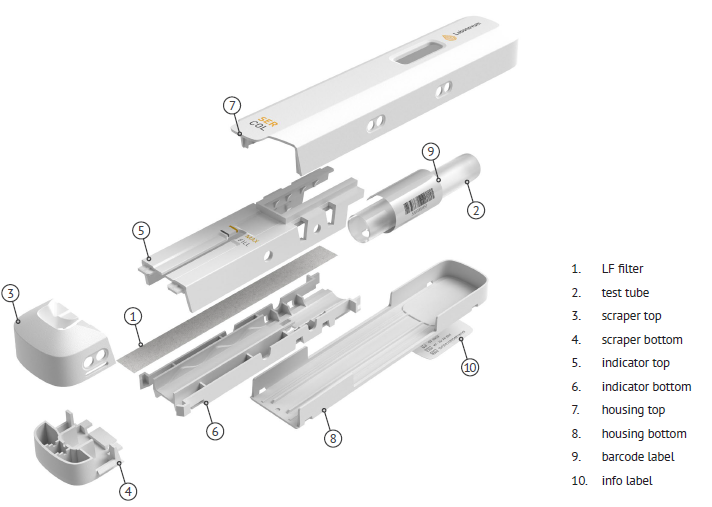
Timeline Labonovum - from idea to product

Pre-kick off meeting
Pre-kick off meeting between Labonovum – Blok System Supply and Alskar design to define the roadmap for the official start in January 2019. (Newspaper article)
Official start SCAUT project
Official start SCAUT project: Labonovum – Blok System Supply and Alskar design define the criteria for Ser-Col for both patient friendliness as well as automated lab processing.
Design strategy
The following point for attention were addressed:
- product architecture
- user environment & configuration
- core meanings tool
- core requirements
- look & feel direction tool
- design & innovation approach
Kick-off meeting
Meeting was held at Labonovum (NL) between the consortium partners Labonovum (NL) – Blok System Supply (NL) – CHU Montpellier (Fr).
Innovation design
Main focus in this fase was research for the best possibilities for automated processing and proof of principle by building a rough model for testing.
Concept design/Prototyping
In this fase the next steps were run through:
- Ideation
- building mockups
- choose direction
- concept modellating
circularity
The most important directions for circularity were:
- reusable parts
Most of the parts used within the new Ser-Col device can either be reused or being shredded and reused within the process of plastic moulding. This is still part of investigation. - biological degradable material
In close consultation with Orange Plastics a study will start towards the use of biological degradable plastics and plastic moulding. This process dependents on the type of mould that will be used for the production of the Ser-Col device.
The new Ser-Col device is officially delivered by Alskar Design
- prototyping
In this important fase of the design both the criteria for patient friendliness, automated processing and circularity comes together. - Designing the new Ser-Col device
Finally a concept is chosen in which the user has to open the Ser-Col by sliding the LF paper containing part out of the outer housing. Blood from a fingerstick can be transferred to the Ser-Col device by scraping off the finger. The indicator level “MAX FILL” indicates when the correct amount of blood is collected. After closing the Ser-Col it is ready for transport to the laboratory by regular postal services.
Ser-Col mold designing is finished
In order to produce Ser-Col on a large scale by plastic molding, a mold has to be designed. Since Ser-Col consists of 10 parts of which at least 7 parts are made of plastic the mold design phase took 2 months longer than expected. The coming 3 months will be used to build to mold.
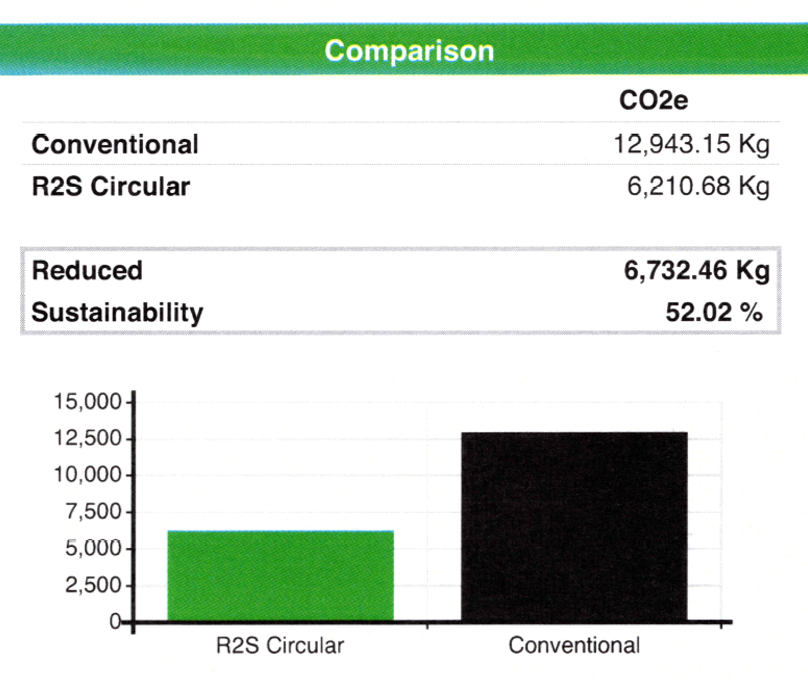
CO2 reduction by circularity
After processing at the laboratory, parts of Ser-Col can be re-used. This so-called return to sender (R2S) process will give a CO2 reduction of more than 50%. Click here to see the CO2 reduction calculation sheet.
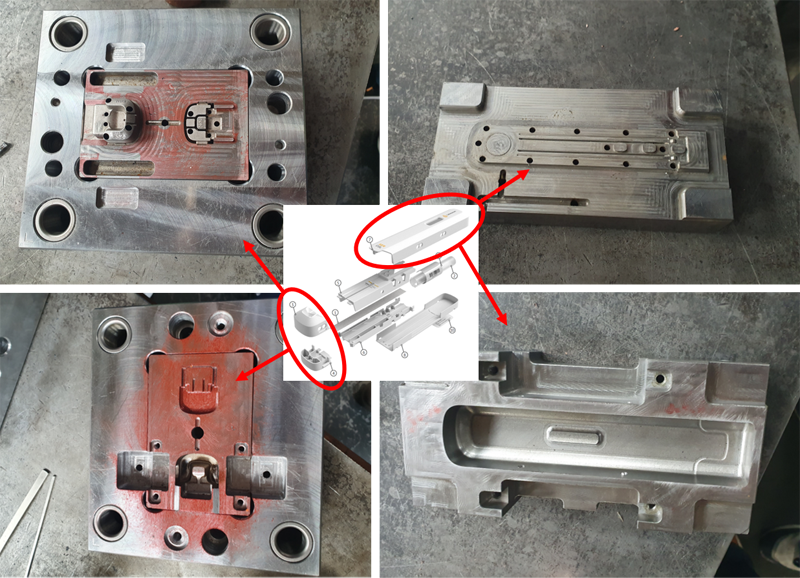
Ser-Col Mold production is Finished
The 7 different parts of Ser-Col are being produced by plastic molding. Liquid plastic is injected under high pressure and high temperature into a steel mold (see pictures). After fast cooling by so-called cooling channel the mold is opened and the plastic parts are released from the mold. After closing, the mold is ready for the next molding step.
The “first out of tools” (FOTs)
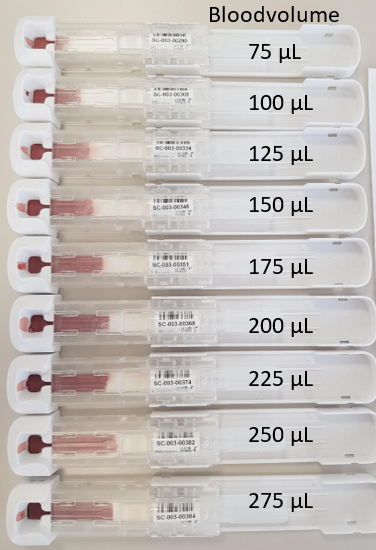
The “first out of tools” (FOTs) is the first production from the mold. With these FOTs we have determined the position of the fill level indicator. The fill level indicator is an opening in the Ser-Col just above the LF paper. Once the blood flows through the fill level indicator, the collected amount of blood is sufficient for testing. Some other minor changes were applied for an optimal automated Ser-Col processing in SCAUT.
Labonovum has received the first SCAUT (Ser-Col AUTomation) from Blok System Supply (BSS).
The last 18 months both Labonovum and BSS has worked intensively on the development of a dried serum spot automated processing solution. From stable dried serum on lateral flow paper to liquid serum in less than 30 seconds!
The coming months will be used to validate SCAUT for processing of dried serum spots for infectious diseases, such as corona.
Impression of the SCAUT processing steps
The first few hundred Ser-Cols are being processed by SCAUT. See this movie for an impression of the different processing steps. Some minor adjustments were necessary to improve the throughput and reduce the turn around time (TAT) to 24 seconds.
See this article in the newspaper about Ser-Col and Corona testing.
Ser-Col Instructions For Use
The instructions for use (IFU) have been produced by video. See this movie that will be provided in different languages.
Validation procedure of SCAUT
Start with validation procedure of SCAUT for serology testing in collaboration with Streeklab Haarlem and Elisabeth-TweeSteden hospital in Tilburg.
First scientific publication Ser-Col in Acta Tropica – Elsevier
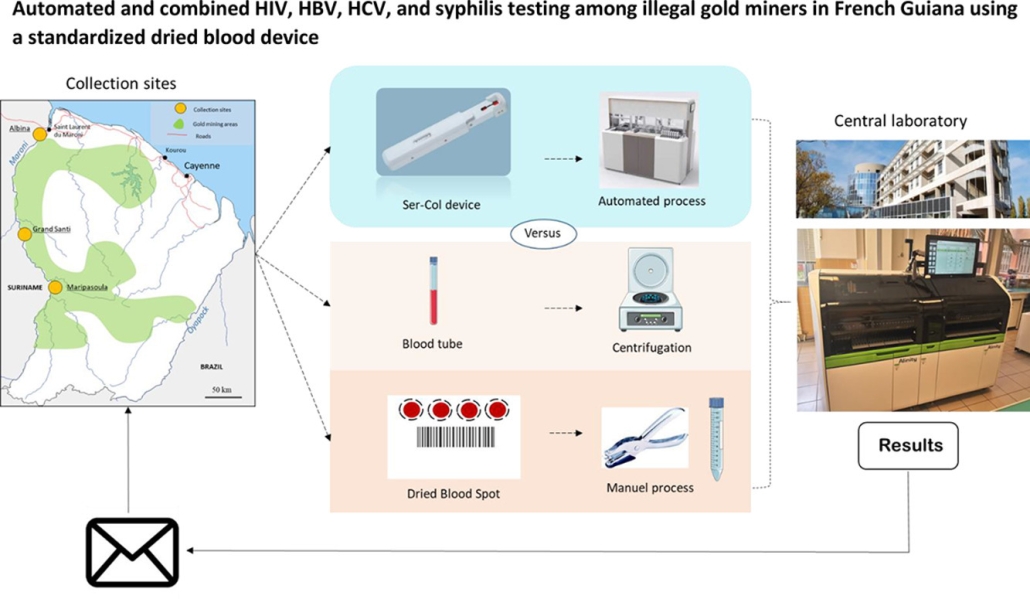
We are pleased to announce the publication ‘Automated and combined HIV, HBV, HCV, and syphilis testing among illegal gold miners in French Guiana using a standardised dried blood device’ in the journal Acta Tropica.
In this publication, Professor Edouard Tuaillon of the Institute of Molecular Genetics of Montpellier (Institut de Génétique Moléculaire de Montpellier (IGMM)) describes the use of the Labonovum Ser-Col blood collection device in comparison with plasma results and DBS results in a study of HIV, HBV, and syphilis among illegal gold miners in French Guiana.
The researchers established a complete concordance of Ser-Col and DBS results for HIV diagnosis compared with plasma results. Ser-Col facilitates large-scale surveys and improves testing of populations living in remote areas.
Interested in reading the entire publication:
https://authors.elsevier.com/c/1g6WL,2UvtImB
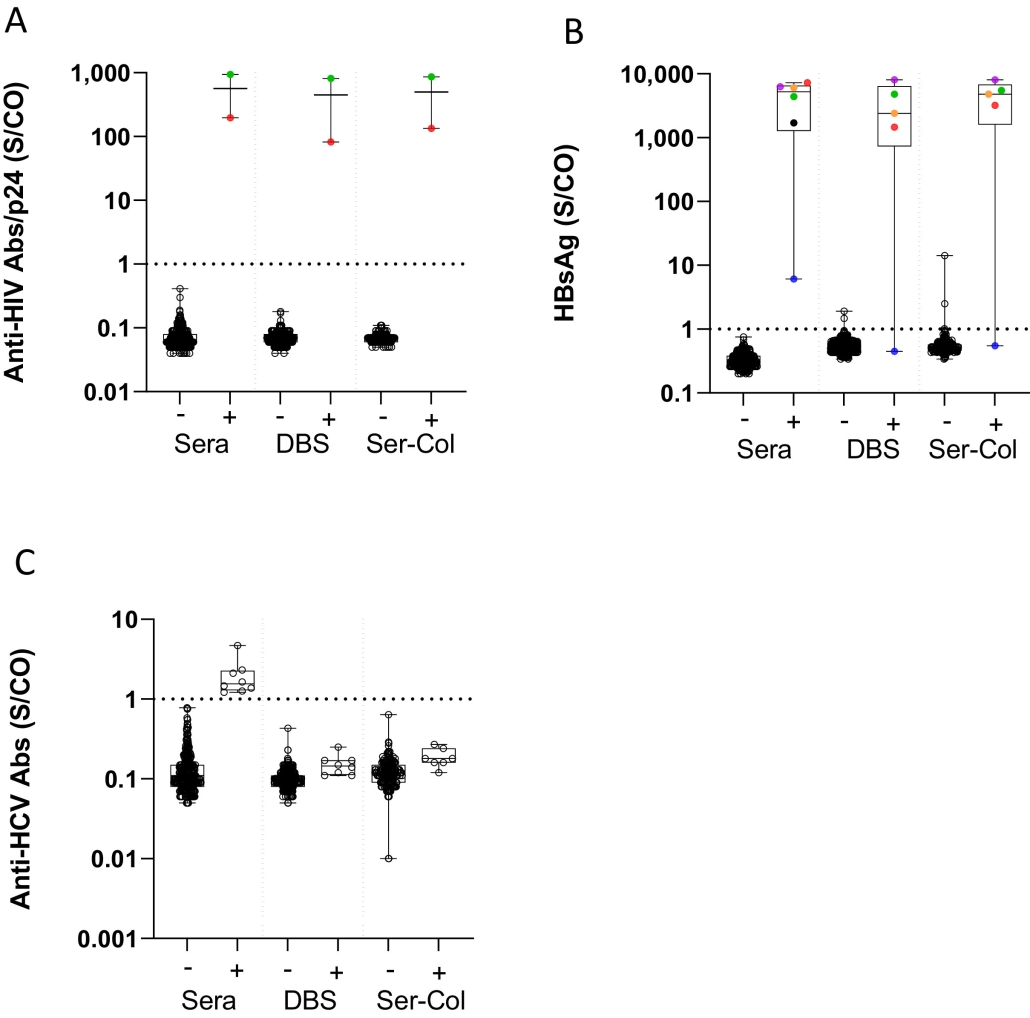
Caption: Results of HIV, hepatitis B and hepatitis C testing on Ser-Col and DBS samples. A) Results of HIV testing in Ser-Col samples and dried blood spot (DBS) according to HIV status in sera. B) Results of HBsAg testing in Ser-Col samples and dried blood spot (DBS) according to HBsAg status in sera. C) Results of hepatitis C antibody testing in Ser-Col samples and dried blood spot (DBS) according to anti-HCV status in sera. All samples tested positive for anti-HCV antibodies were negative for HCV RNA.
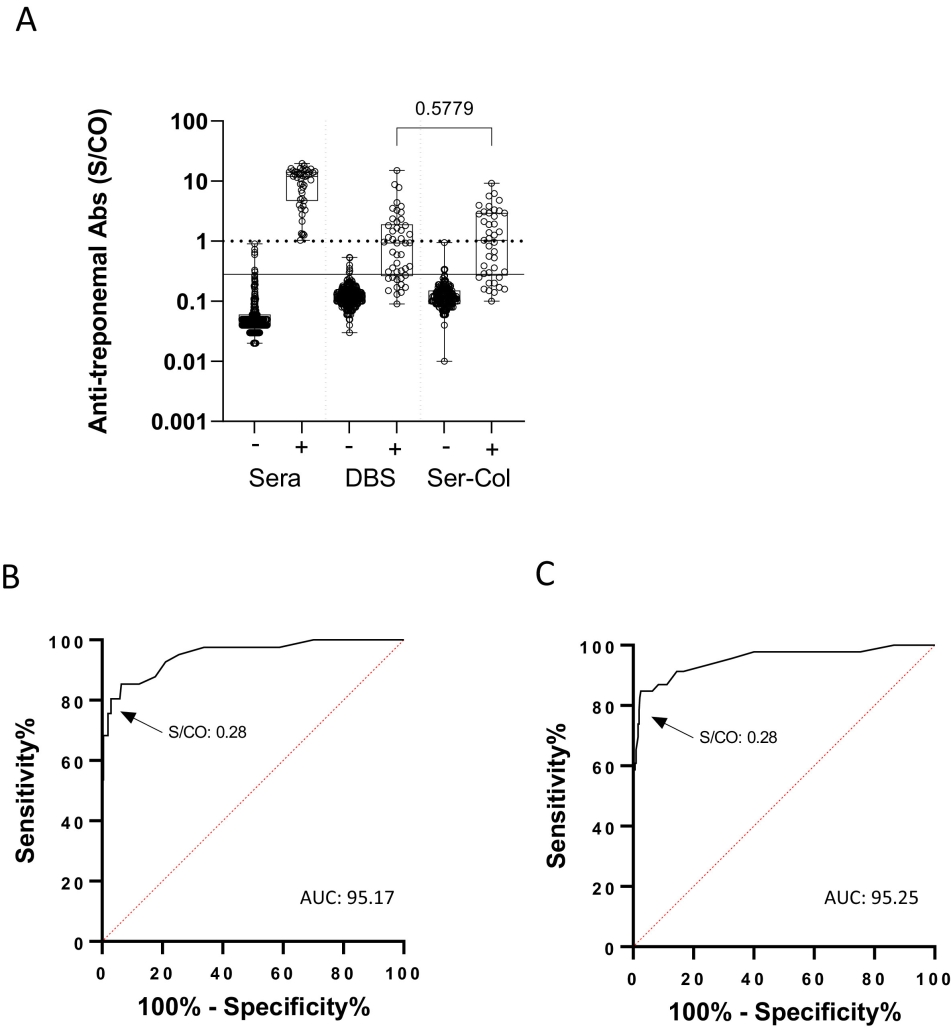
Caption: Anti-treponemal antibody results in Ser-Col and Dried Blood Spot samples. A) Anti-treponemal antibodies in Ser-Col samples and dried blood spot (DBS) according to syphilis serological status in plasma; dotted line: threshold of the manufactuer; solid line: optimized threshold for Ser-Col and DBS samples. B) Receiver operating characteristic curve (ROC) evaluating anti-treponemal antibody detection in Ser-Col samples. C) ROC evaluating anti-treponemal antibody detection in DBS samples.